VADUZ, Liechtenstein, 16 December, 2021 – PerioTabs®, a home-care gum and teeth brushing solution based on bonyf’s non-antibiotic biofilm removal formulation NitrAdine®, was recently evaluated for its clinical efficacy in 60 gingivitis patients.
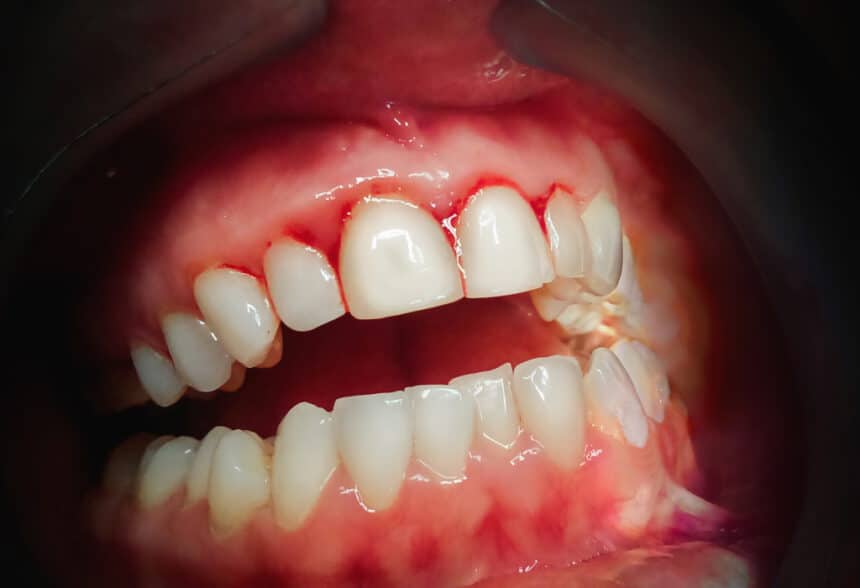
Results of the study showed that a clear improvement in the reduction of bleeding gums occurred in 90% of the patients using PerioTabs®. 50% of the PerioTabs® users showed a reduction in plaque formation. Visual inspection of the gums showed a well-defined improvement of the gum tissue state in terms of colour, firmness and attachment to teeth. No side effects were reported.
In addition, 76% of the PerioTabs® group reported a sensation of “freshness” in the mouth and the satisfaction rate related to the use of PerioTabs® was very high.
Due to the interest to search for alternatives to chlorhexidine, the clinical study was conducted by the CIR-Dental School, University of Torino, Italy, in collaboration with periodontologists at Studio Sicor (Torino). All 60 selected subjects underwent regular prophylaxis and professional oral care at baseline. After 30 days the subjects were recalled for measurement of bleeding and plaque index. 30 patients were instructed to use PerioTabs® for 10 days. The remaining 30 patients were instructed to perform their usual oral hygiene regimen. 15 days later, the clinical parameters were re-evaluated in all 60 patients.
Gingivitis (also known as bleeding gums) is the most common disease in the world affecting 90% of the population. If untreated, gingivitis will lead to periodontitis, the main cause of tooth loss. Current home-based treatments to manage gingivitis are based on the use of chlorhexidine. Chlorhexidine usage however, comes with many side effects including extrinsic staining, calcus formation, taste alterations and increasing bacterial resistance.
The study was published in the European Journal of Dentistry, December 2021.
For more details: https://pubmed.ncbi.nlm.nih.gov/34875712/
A copy of the full publication is available on request.